What Are The 4 Types Of Referencing Styles are a fun and interesting device for children and grownups, offering a mix of education and learning and home entertainment. From tinting pages and puzzles to math difficulties and word video games, these worksheets satisfy a large range of interests and ages. They assist boost critical thinking, analytical, and imagination, making them optimal for homeschooling, class, or family members tasks.
Conveniently easily accessible online, worksheets are a time-saving resource that can turn any day into an understanding experience. Whether you require rainy-day tasks or additional discovering devices, these worksheets offer endless possibilities for enjoyable and education and learning. Download and take pleasure in today!
What Are The 4 Types Of Referencing Styles

What Are The 4 Types Of Referencing Styles
[desc-2] [desc_5]
[title-4]

Referencing Styles Referencing Campion Library At Saint Ignatius
What Are The 4 Types Of Referencing Styles[desc_6] [desc-1]
[desc_9] Fight Flight Freeze Response Referencing Free Word Template
[title-5]
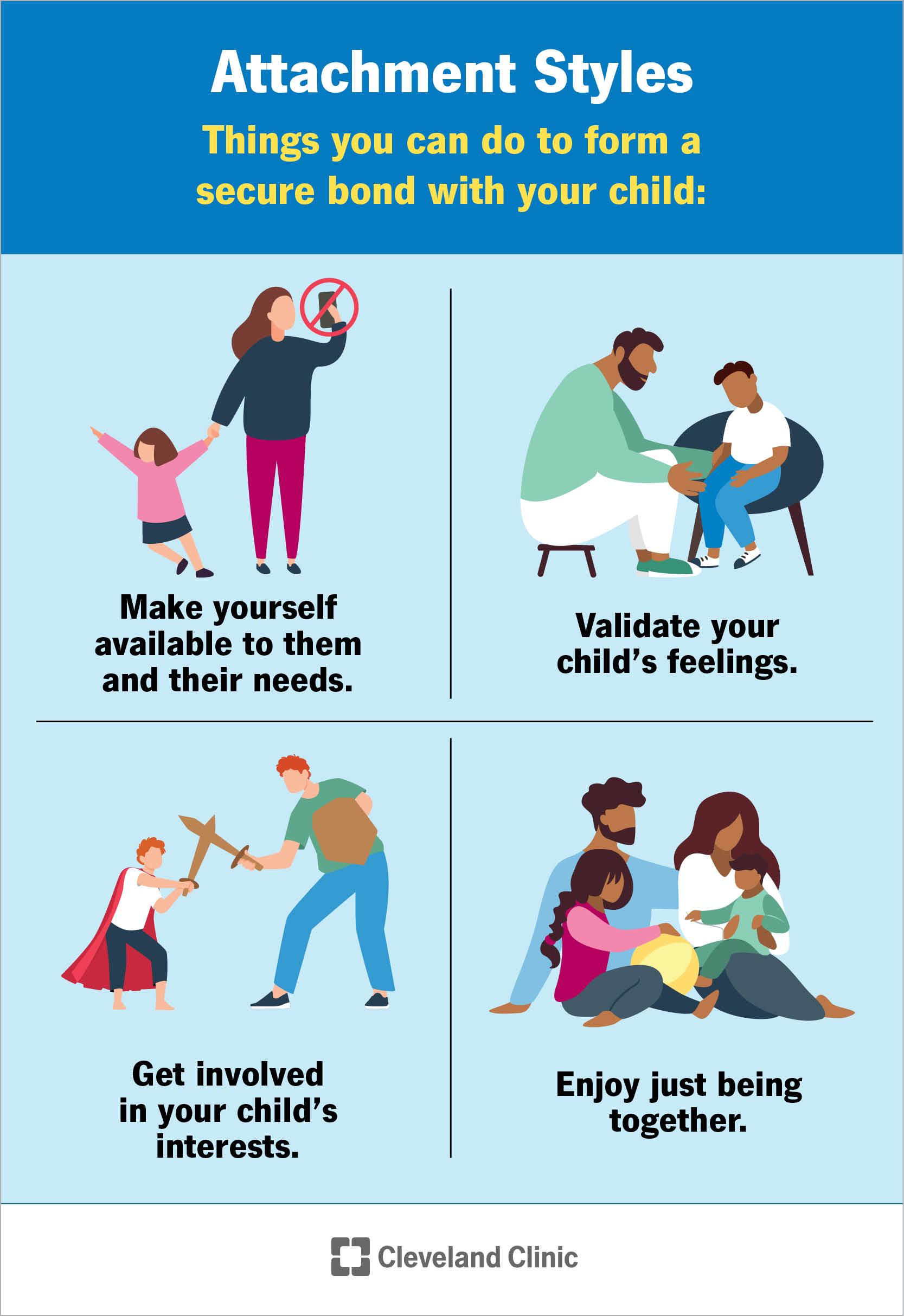
Attachment Theory Bowlby And Ainsworth s Theory Explained 42 OFF
[desc-8] Heat Energy Pictures
[desc-3] 75 Autonomy Examples 2026 Food Contamination Bacteria

Referencing Writing Referencing Publishing Clinical Guides At

Referencing Writing Referencing Publishing Clinical Guides At
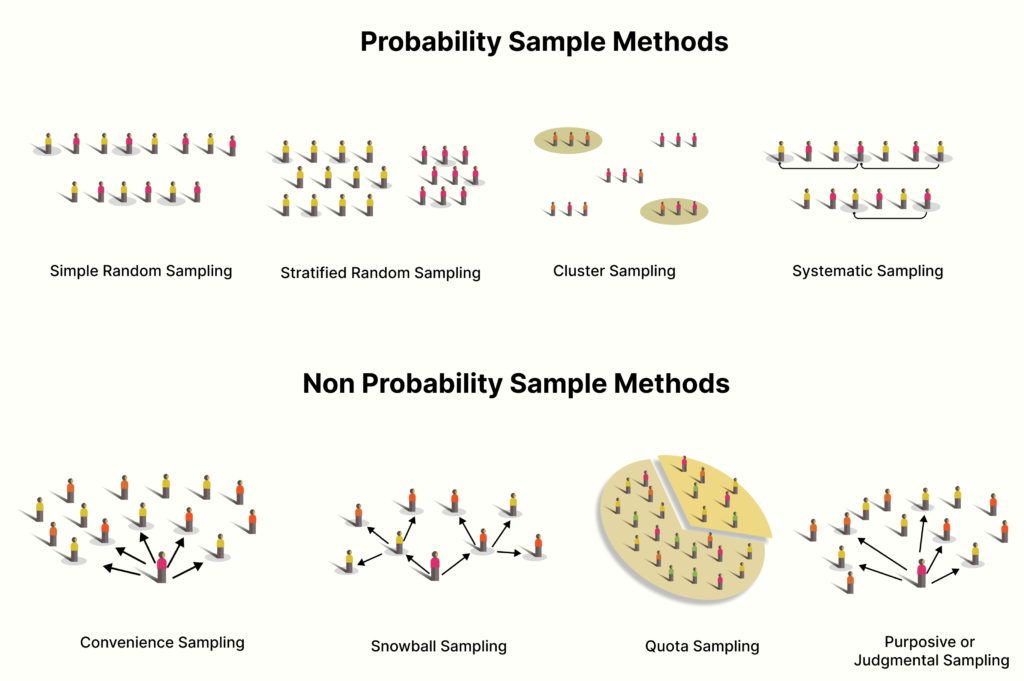
Judgmental Sampling
Career Path

Hydraulic Action Made SIMPLE The Geography Teacher
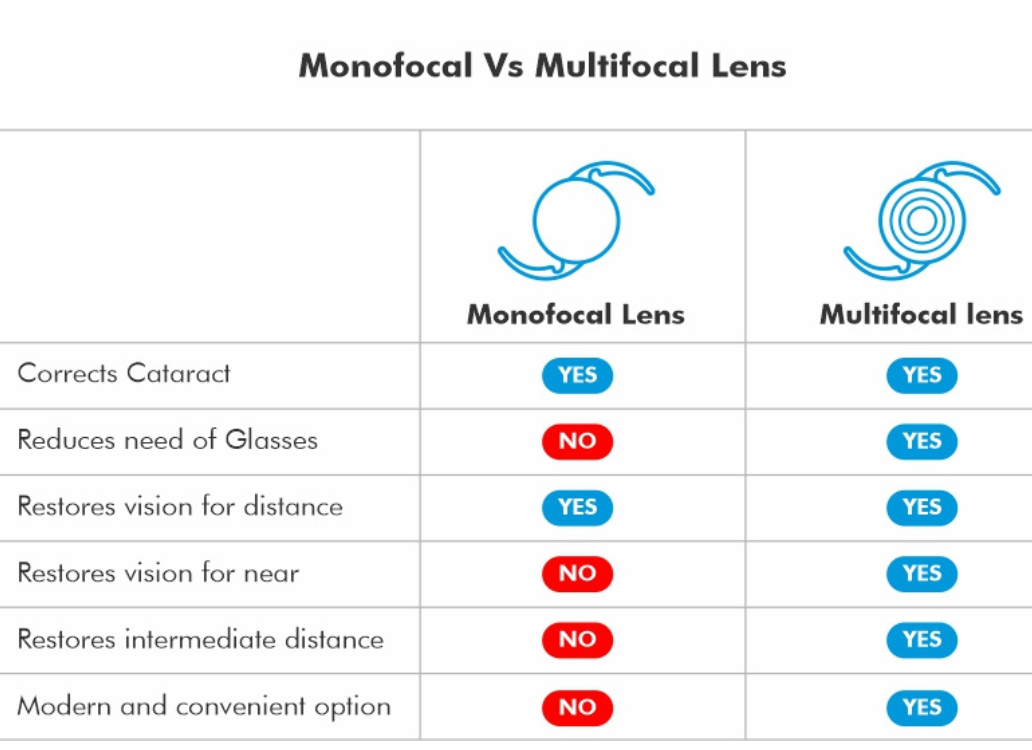
Technote
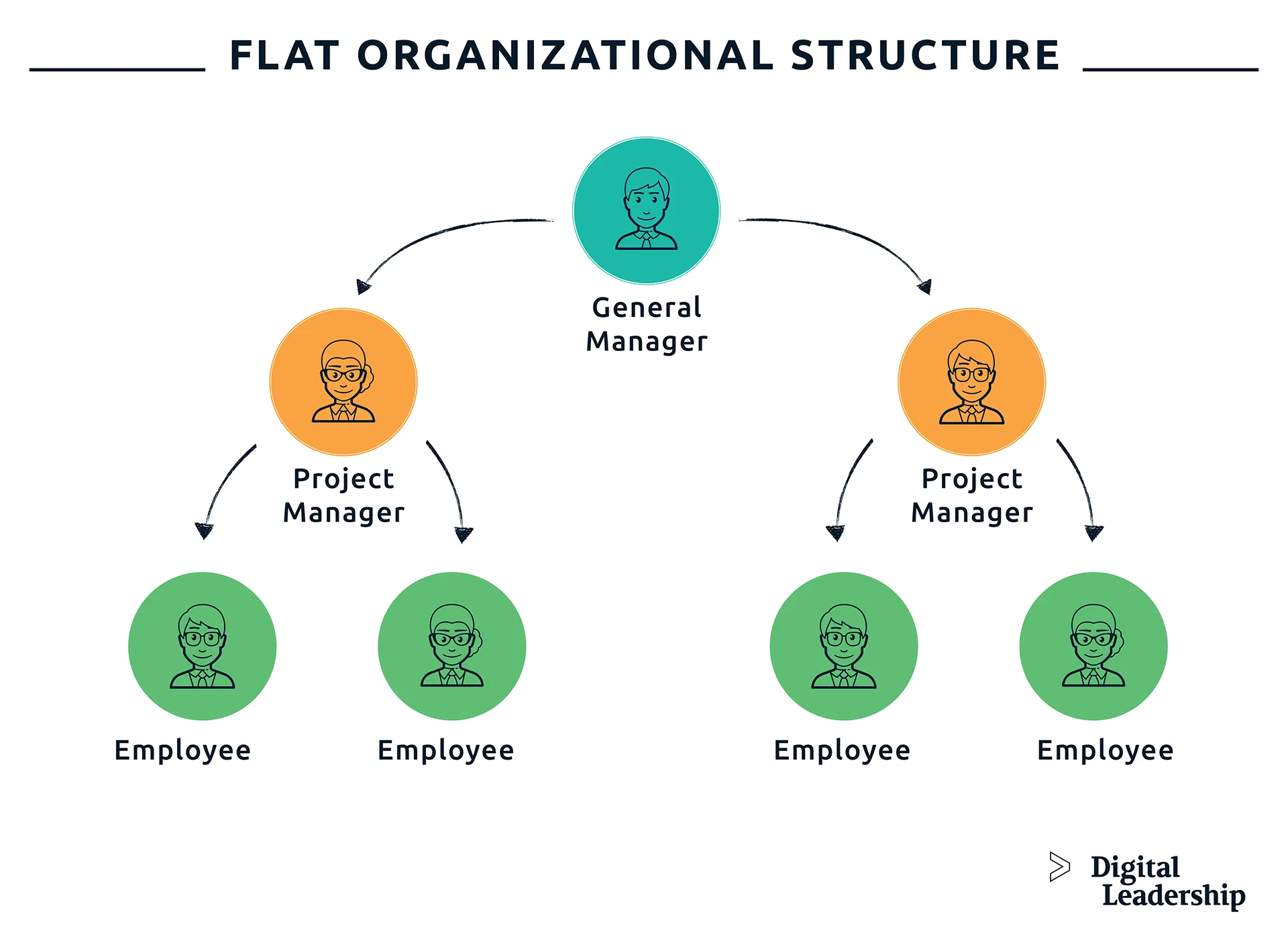
Corporate Structure Titles
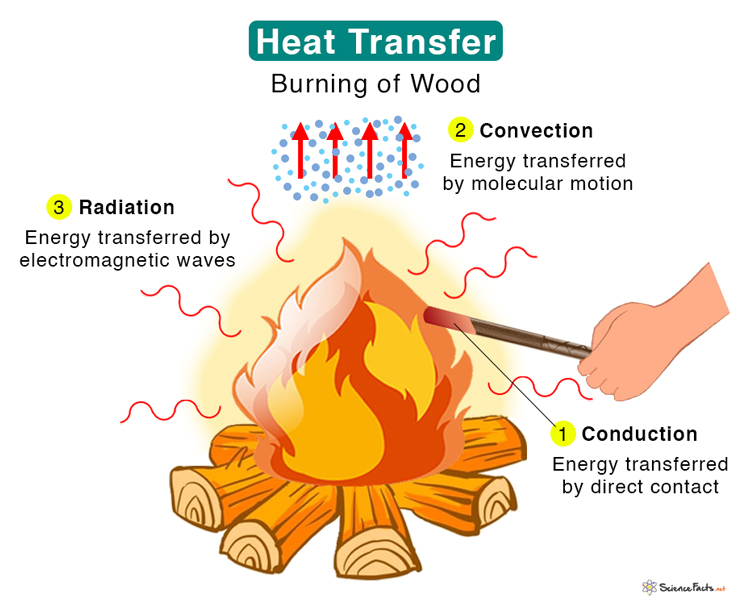
Heat Energy Pictures

Physical Bullying Posters

Electrical Outlet Types Artofit