Heaviside Function Laplace Examples are an enjoyable and interesting tool for children and grownups, offering a blend of education and learning and amusement. From coloring pages and problems to math difficulties and word games, these worksheets accommodate a wide variety of passions and ages. They help improve essential thinking, problem-solving, and creativity, making them suitable for homeschooling, class, or family members tasks.
Easily easily accessible online, worksheets are a time-saving source that can turn any day into an understanding experience. Whether you need rainy-day tasks or extra discovering tools, these worksheets supply endless possibilities for fun and education and learning. Download and appreciate today!
Heaviside Function Laplace Examples
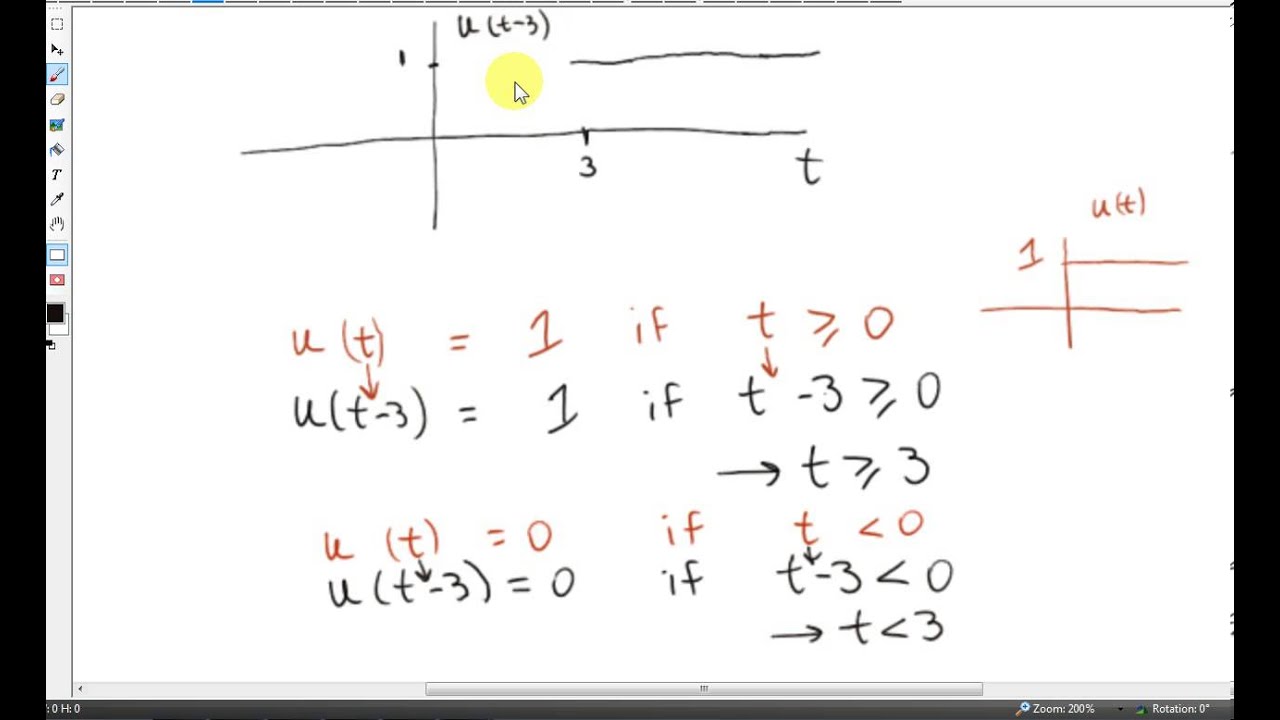
Heaviside Function Laplace Examples
Using the activity series table answer the following questions 2 Using the activity series how do you decide if a reaction will or will not take place between two species 3 Describe what you would see when a piece of Mg metal is placed in an aqueous solution of copper II sulfate Identify each reaction as either single replacement or double replacement and then balance the reactions. 1. Zn + HCl ZnCl 2 + H 2 Type of reaction: _____ 2. Ca 3 N 2 + H 2 O Ca(OH) 2 + H 3 N Type of reaction: _____ 3. KI + Pb(NO 3) 2 KNO 3 + PbI 2 Type of reaction: _____ 4.
Single Replacement Reactions Vancouver Community
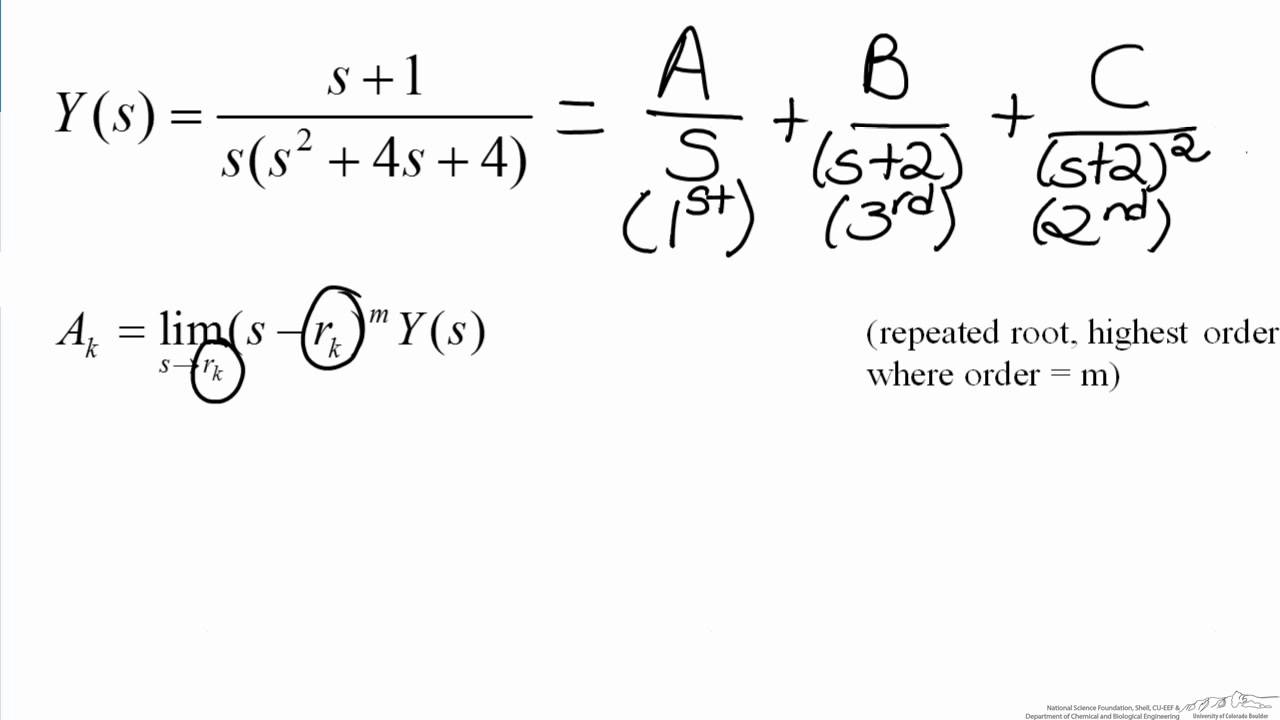
Laplace Transforms Heaviside Method YouTube
Heaviside Function Laplace ExamplesSingle Replacement Reaction Worksheet For each reaction use the activity series to complete the reaction. If no reaction will occur, write “no reaction”. Make sure you balance your final answer. 1. Ag + KNO 3 → 2. Zn + AgNO 3 → 3. Cl 2 + KI → 4. Cu +. Worksheet 4 Single Replacement Reactions Step 1 Write the formulas of the reactants on the left of the yield sign Step 2 Look at the Activity Series on page 333 to determine if the replacement can happen
In these reactions, a free element reacts with a compound to form another compound and release one of the elements of the original compound in the elemental state.There are two different possibilities: 1. One cation (+ ion) replaces another. 2. One anion (- ion) replaces another. REACTION GUIDELINES REACTION FORMAT REACTION DESCRIPTION REACTION . Ex Find The Laplace Transform Of A Step Function method 1 YouTube Laplace Transform Of Piecewise Functions Using Heaviside Function YouTube
Single And Double Replacement Reactions Practice WS

Laplace Transform Involving Heaviside Functions YouTube
An answer key is provided with the balanced equations or NR if no reaction occurs This document provides a chemistry worksheet on single replacement reactions It contains 20 chemical equations to balance with possible products based on an activity series reference chart Laplace Transform Method 2 Heaviside Functions Differential
Complete each word equation write formulas and balance the reaction equation Then identify and place the type of reaction single replacement or double replacement in the blank provided Laplace Transform Of A Piecewise Function Unit Step Function YouTube 3 3 Transformada De Laplace Fun o De Heaviside YouTube

The Unit Step Function YouTube
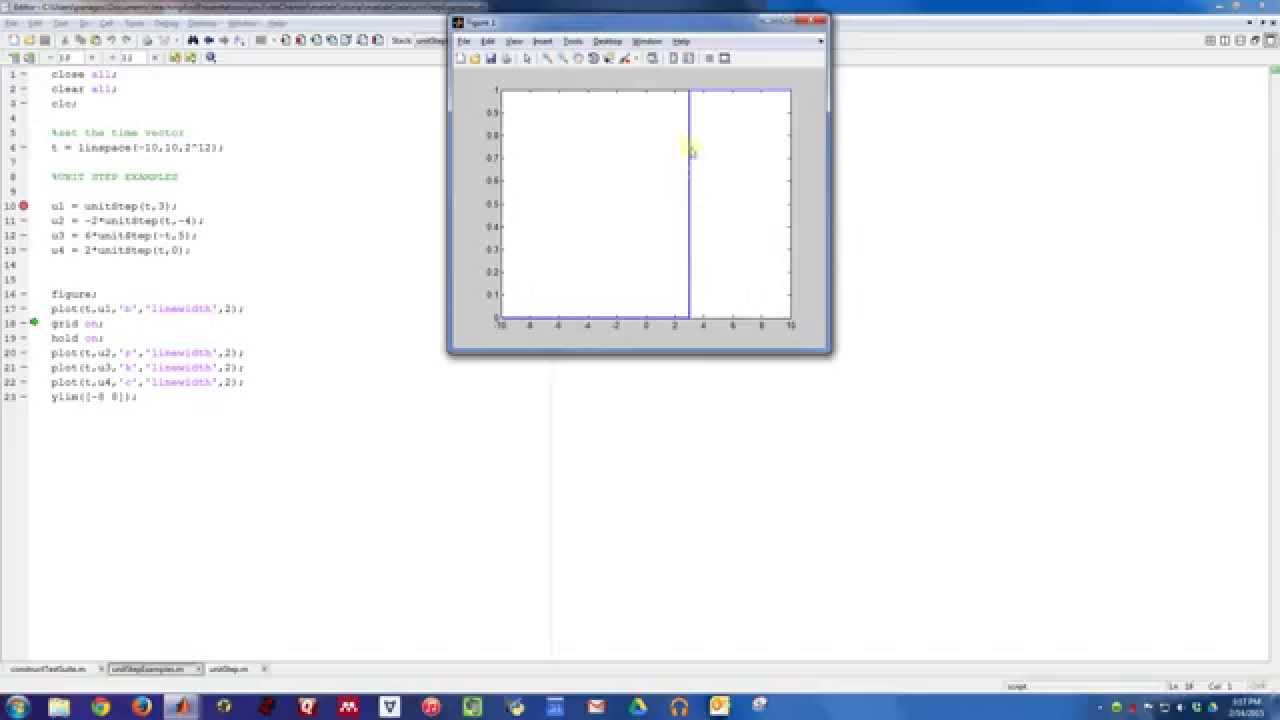
Matlab Examples The Unit Step Function YouTube
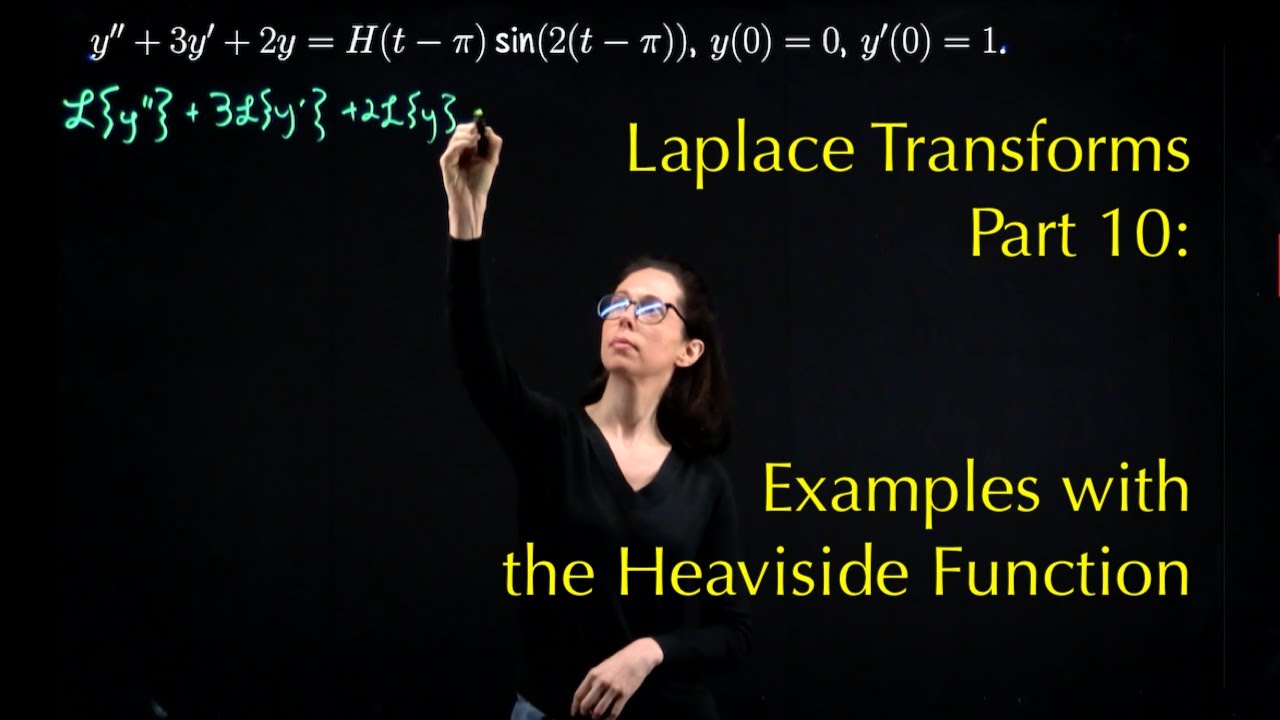
Laplace Transforms 10 Examples With The Heaviside Function YouTube

Unit Step Functions Heaviside Function Laplace Transform

Laplace Transform Of The Heaviside Function YouTube

Heaviside Function In Differential Equations YouTube

Inverse Laplace Transforms As Convolutions YouTube

Laplace Transform Method 2 Heaviside Functions Differential

Inverse Laplace Transform Laplace Inverse Transform Formulas Solved

Laplace Transform Of Heaviside And Dirac Delta Functions And Shift