Class 6th Ncert Science Solutions Extra Questions are a fun and engaging tool for children and adults, providing a blend of education and learning and amusement. From coloring pages and puzzles to mathematics difficulties and word video games, these worksheets satisfy a vast array of interests and ages. They aid improve important thinking, analytical, and creativity, making them ideal for homeschooling, classrooms, or family tasks.
Quickly easily accessible online, printable worksheets are a time-saving resource that can turn any day right into a learning journey. Whether you require rainy-day tasks or supplemental knowing devices, these worksheets provide endless possibilities for enjoyable and education and learning. Download and install and enjoy today!
Class 6th Ncert Science Solutions Extra Questions

Class 6th Ncert Science Solutions Extra Questions
Using the activity series table answer the following questions 2 Using the activity series how do you decide if a reaction will or will not take place between two species 3 Describe what you would see when a piece of Mg metal is placed in an aqueous solution of copper II sulfate Identify each reaction as either single replacement or double replacement and then balance the reactions. 1. Zn + HCl ZnCl 2 + H 2 Type of reaction: _____ 2. Ca 3 N 2 + H 2 O Ca(OH) 2 + H 3 N Type of reaction: _____ 3. KI + Pb(NO 3) 2 KNO 3 + PbI 2 Type of reaction: _____ 4.
Single Replacement Reactions Vancouver Community
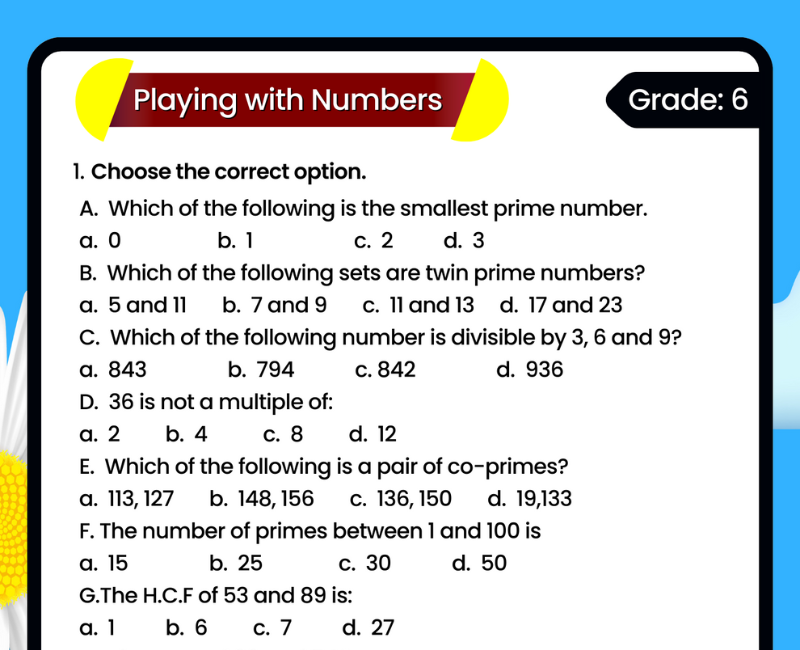
Free Worksheet For CBSE Grade 6 Maths 2
Class 6th Ncert Science Solutions Extra QuestionsSingle Replacement Reaction Worksheet For each reaction use the activity series to complete the reaction. If no reaction will occur, write “no reaction”. Make sure you balance your final answer. 1. Ag + KNO 3 → 2. Zn + AgNO 3 → 3. Cl 2 + KI → 4. Cu +. Worksheet 4 Single Replacement Reactions Step 1 Write the formulas of the reactants on the left of the yield sign Step 2 Look at the Activity Series on page 333 to determine if the replacement can happen
In these reactions, a free element reacts with a compound to form another compound and release one of the elements of the original compound in the elemental state.There are two different possibilities: 1. One cation (+ ion) replaces another. 2. One anion (- ion) replaces another. REACTION GUIDELINES REACTION FORMAT REACTION DESCRIPTION REACTION . Ncert Maths Grade 9 Textbook Impianto Solare Termico Come Funziona Enel X 55 OFF
Single And Double Replacement Reactions Practice WS

NCERT Exemplar Solutions For Class 6 Science Chapter 1 Food Where
An answer key is provided with the balanced equations or NR if no reaction occurs This document provides a chemistry worksheet on single replacement reactions It contains 20 chemical equations to balance with possible products based on an activity series reference chart NCERT Exemplar Solutions For Class 6 Science Chapter 6 Changes Around
Complete each word equation write formulas and balance the reaction equation Then identify and place the type of reaction single replacement or double replacement in the blank provided NCERT Class 6 Curiosity Science Book 2024 Edition StudyUpIndia Class 6th NCERT SCIENCE BPSC TRE 4 SCIENCE BPSC TRE 4 bpsctre4

Extra Questions Of Chapter 10 Class 7 Science Free Math Worksheet

Ncert Science Class 9 Textbook

Class 6th Ncert Science Book Lession 1st Part 3 YouTube

Class 6th Ncert Science Bihar Board Chapter 1 Questions Answers
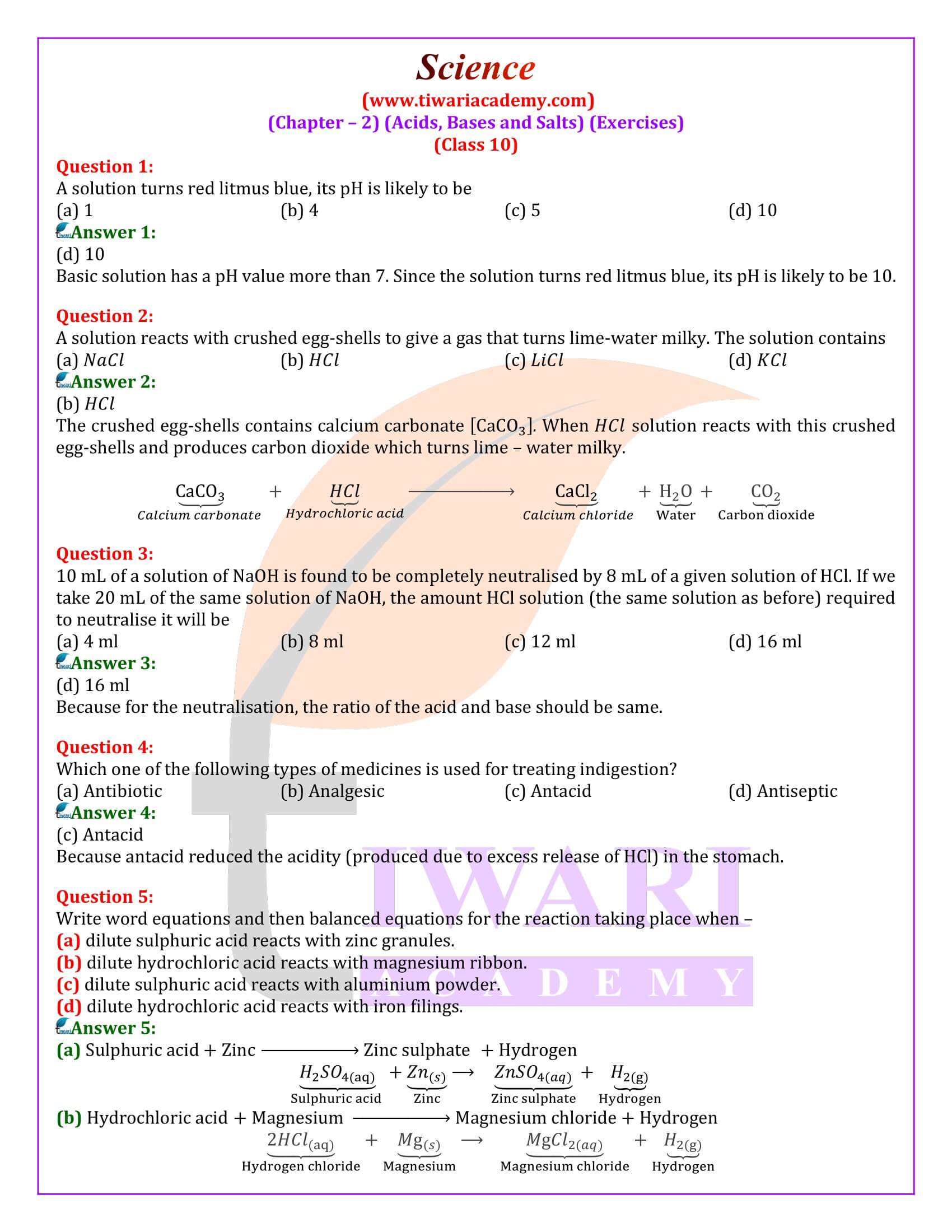
Class 10 Science Chapter Notes Of Acids Bases And Salts 58 OFF
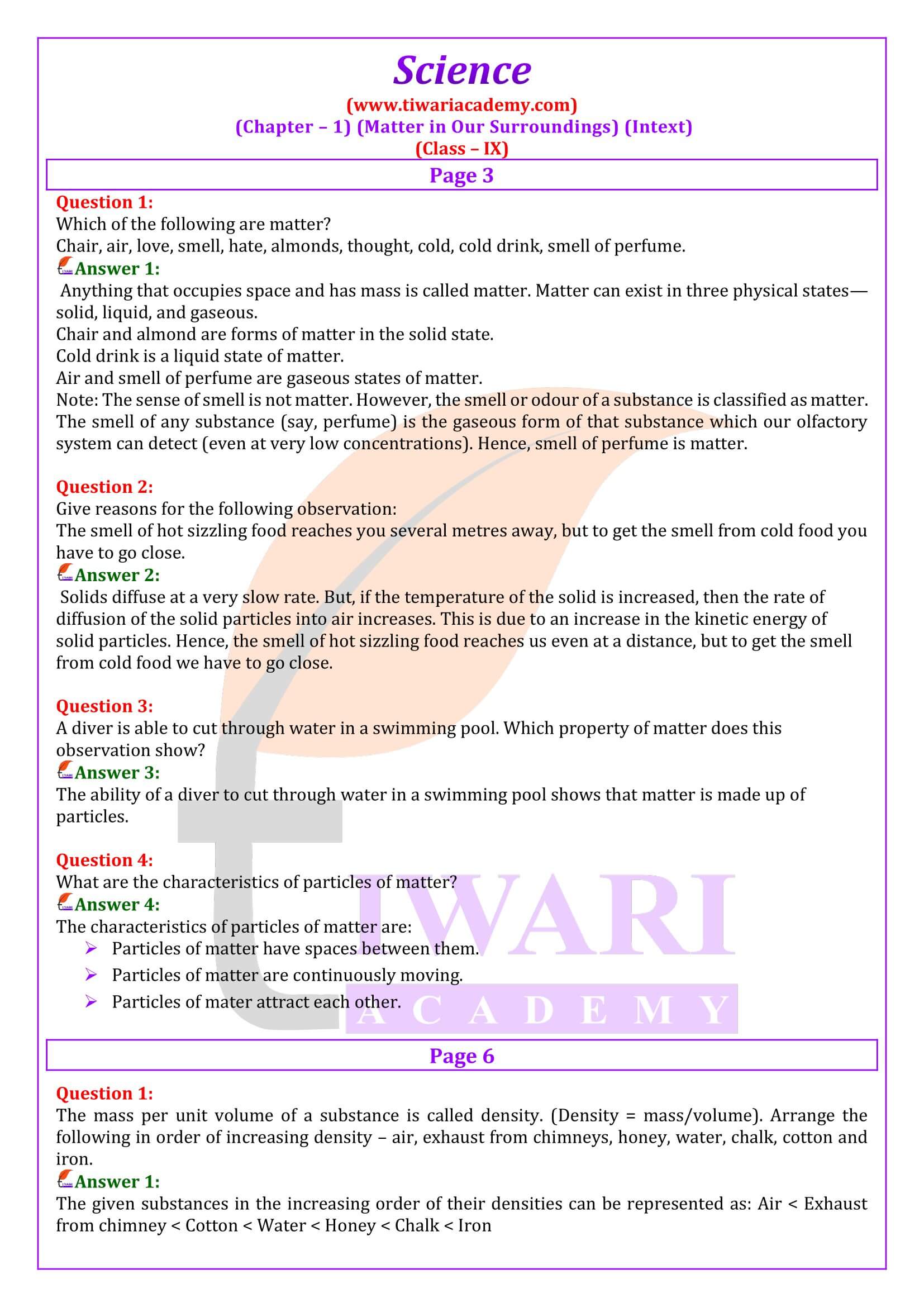
Is Matter Around Us Pure Class 9th Science Handwritten 43 OFF

NCERT Exemplar Solutions For Class 6 Science Chapter 1 Food Where

NCERT Exemplar Solutions For Class 6 Science Chapter 6 Changes Around

Class 6th NCERT Science First Chapter Components Of Food 10 Most
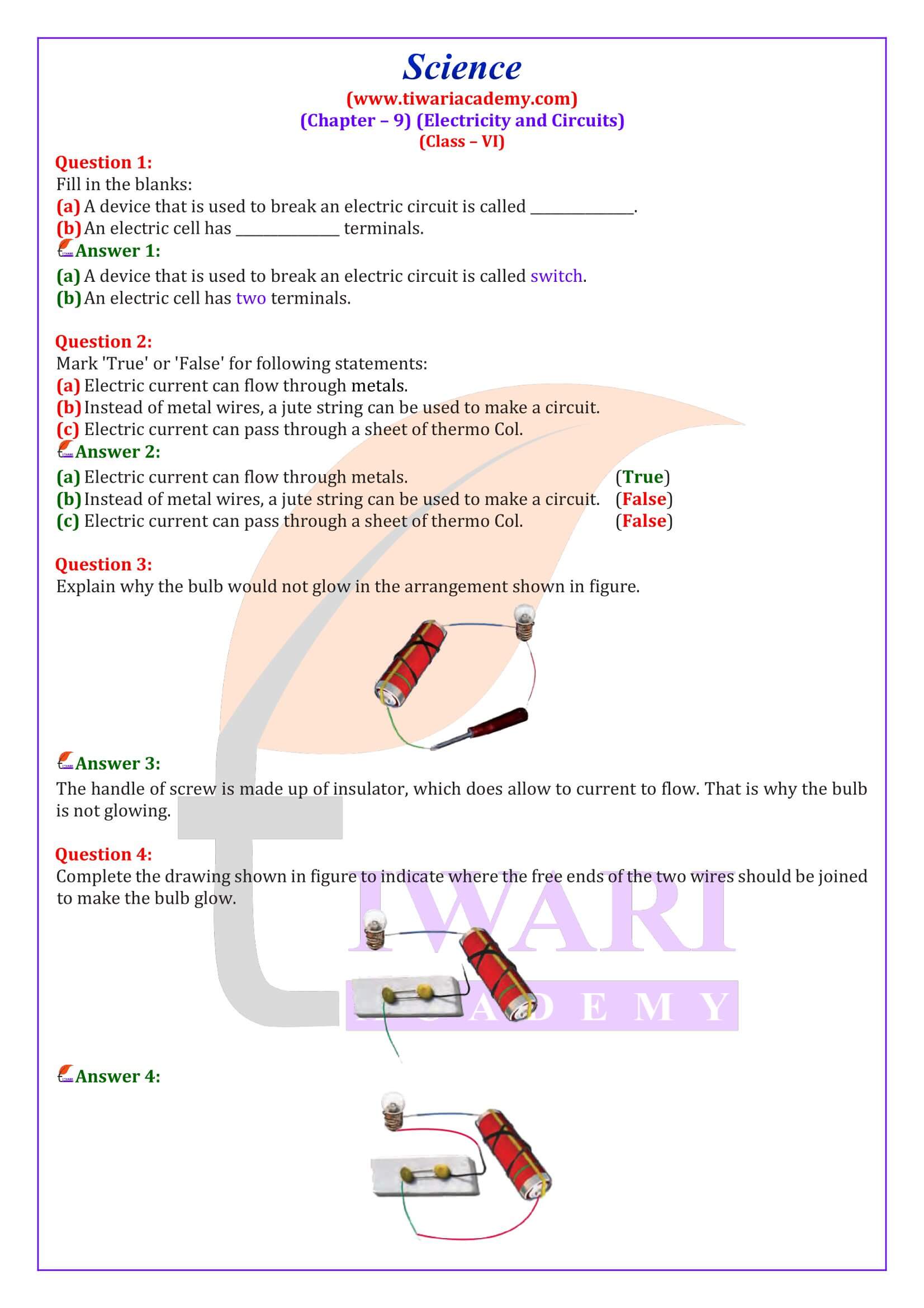
50 Examples Of Prefixes And Suffixes Definition And 52 OFF